“CASS Transformation and Research Lab“ – Group Christian Lamm
As part of the CASS-project, the CASS Transformation and Research Lab works on the development of in vitro methods enabling genetic transformation of agriculturally relevant Cassava genotypes, and routinely provides our project partners with genetically modified Cassava plants.
Plant biotechnology is indispensable for modern plant research and breeding. For instance, it can be used to elucidate the molecular functions of enzymes and proteins, improve existing metabolic pathways, or introduce entirely new ones. In many cases, even modern genome editing techniques such as the CRISPR/Cas system rely on methods of classical genetic engineering.
However, the lengthy procedures require precise adaptation to the plant of interest. In fact, even within a species, only few cultivars may be transformed efficiently. A major disadvantage, especially when it comes to genetic modification of crop plants: Accessible cultivars do not necessarily meet agricultural needs, for example due to lower yields or higher susceptibility to pathogens or abiotic stress. The emergence of genome editing techniques has particularly led to increased research efforts aiming at transformation of agriculturally useful varieties of various crops.
In times of global climate change and increasing population, plant biotechnology alongside modern breeding techniques could make a crucial contribution to urgently needed global food security.
Within the CASS project, we are addressing this issue with the tropical crop Cassava, which is mainly grown by smallholder farmers in Sub-Saharan Africa for its starchy storage roots. However, drastically lower yields are achieved here compared to other regions, mainly due to the lack of availability of fertilizers, biocides, and modern agricultural equipment. Therefore, our stated goal is a biotech-driven yield stabilization in low input environments.
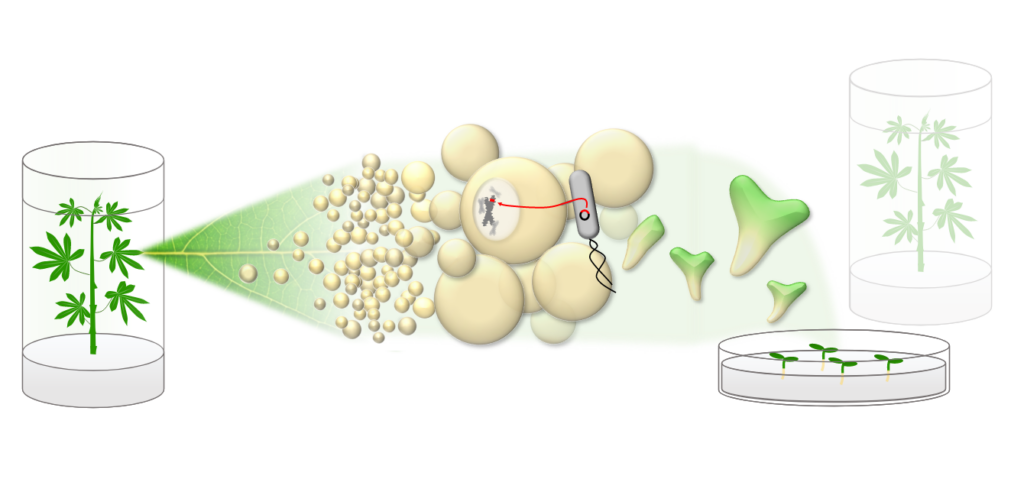

The CASS Transformation and Research Lab is aiming to improve the genetic transformation of Cassava in order to be able to modify farmer-preferred varieties. We investigate chemical media additives that alter the developmental program of plant cells (e.g. by targeting the epigenetic regulation) and thus enable transformation and/or regeneration.
Additionally, we work on genetic elements influencing the transformation and regeneration processes, as the ectopic expression of certain developmental regulators can be used to genetically modify cultivars that were previously not amenable to transformation – a principle that other research groups have already applied successfully in crops such as maize.

In addition to these research questions, we perform routine transformations of the Cassava model genotype TMS60444 for our project partners, covering the entire workflow (Fig.1): Cassava plants are grown under sterile conditions and treated with phytohormones to produce dedifferentiated callus tissue.
A suitable transformation plasmid is generated through molecular cloning and introduced into a lab strain of the soil bacterium Agrobacterium tumefaciens. The bacterium is naturally capable of introducing genetic material into plant cells, and through cultivation with the callus tissue, targeted transfer of the desired DNA can occur.
By altering the media composition, fully formed plants with the desired genetic material integrated into their own genome can be regenerated (Fig.2). After molecular confirmation of genomic integration, the plants are vegetatively propagated and can be analyzed further (Fig.3,4).

