“Cassava Source-Sink“ – Group Wolfgang Zierer

We strive to improve cassava storage root yield. Cassava is a tropical crop that is consumed by over 800 million people worldwide and is of special importance for the food security of smallholder farmers in Sub-Saharan Africa.
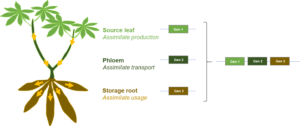
The plant grows up to 3-4 meters in height and generates several starchy storage roots under good conditions. These storage roots (Fig. 1) and their starch are the basis for a multitude of foods.

Alongside many other options, the storage roots can be consumed as vegetables in cooked or fried state or their starch can be processed into a variety of products, like the African Gari, a popular kind of puree.
IUnfortunately, cassava yield is still comparably low in contrast to the heavily yield-optimized crops like wheat or maize. A sustainable cassava yield improvement would therefore have a large impact on the food security of millions of people. Therefore, we dedicate our work towards cassava yield improvement.
This goal is unfortunately not within easy reach and a biotechnological improvement of cassava yield is dependent on a very detailed understanding of the plant. Therefore, our work also contains a lot of basic science to unravel the physiological and biochemical processes of this exiting plant species.

We mainly research the regulation of carbohydrate metabolism in auto- and heterotrophic tissues, assimilate transport, as well as developmental processes. Within these processes, we are trying to identify genes with positive impact on storage root yield and to combine them eventually for even larger impact (Fig. 3).

We test our target genes via transgenic cassava plants, which we generate ourselves or with the help of our collaboration partner at the ETH Zurich. Cassava transformation comprises refined tissue culture protocols, which can regenerate entire plants from dedifferentiated plant cells (Fig. 4).
As a first step, we test our modified cassava plants with various techniques in the greenhouse. Plants that can outperform their unmodified genetic background are of course of special interest for us (Fig. 5).

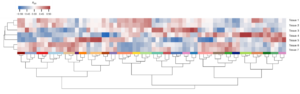
In addition to the determination of agronomic parameters and different physiological experiments like gas-exchange measurements (Fig. 6), we routinely employ most modern techniques of molecular plant research, like transcriptome- and proteome studies (Fig. 7).
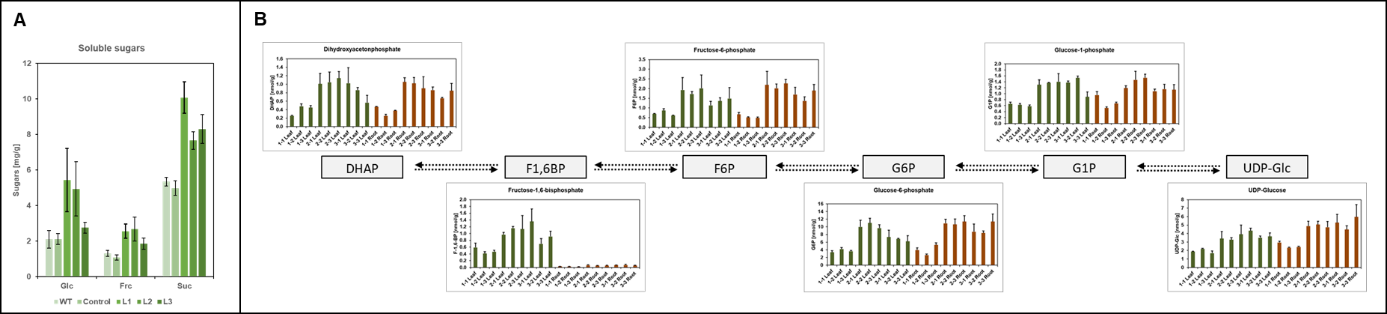
Several metabolite measurements are part of our routine application as well. Enzymatic or chromatographic sugar measurements for instance, grant us insight into primary plant metabolism (Fig. 8).
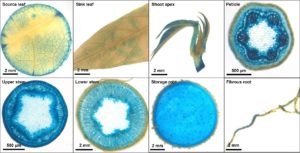
In addition to the identification and analysis of various genes, we also work on the improvement of tools for the generation of better transgenic cassava plant. Examples are better transformation protocols or the identification of tissue specific promoter sequences (Fig. 9)
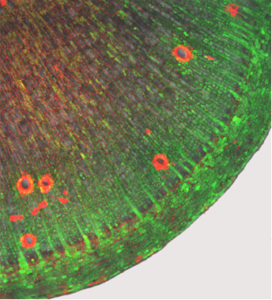
Furthermore, we conduct different studies on cassava wild type plants and various breeding material to understand underlying physiological processes, like the storage root formation or the assimilate transport.
Recently, we could clarify the symplasmic connections in cassava stems and storage roots by introducing and following fluorescent substances into the plant (Fig. 10).
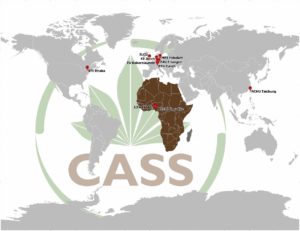
Our research group is part of the Cassava Source-Sink (www.cass-research.org) project (CASS) and has excess to a large network of plant science techniques and expertise through our various partners (Fig. 11).
We have close collaborations with:
- International Institute of Tropical Agriculture (IITA), Bioscience, Ibadan, Nigeria
- National Root Crops Research Institute (NRCRI), Umudike, Nigeria
- Chung-Hsing University (NCHU), Advanced Biotechnology Center, Taichung, Taiwan
- Sainsbury Laboratory Cambridge University (SLCU), Cambridge, United Kingdom
- Boyce Thompson Institute, Plant Research (BTI), Ithaca NY, USA
- Eidgenössische Technische Hochschule (ETH), Chair of Biochemistry, Zurich, Switzerland.
- Technische Universität Kaiserslautern, Chair of Plant Physiology, Germany

Fig. 12: Different phases of field-testing at NCHU Taichung, Taiwan. - Forschungszentrum Jülich, Institut für Bio- und Geowissenschaften, Germany
Together with our partners, we established an entire plant-testing pipeline, starting with the generation of transgenic cassava plants, followed by laboratory and greenhouse pre-testing, and ultimately field-testing (Fig. 12).
Overview of a young cassava field
Overview of an advanced cassava field

Our field trials are executed together with NCHU Taichung, Taiwan and the IITA Ibadan, Nigeria (Fig. 13).
We test transgenic cassava plants with alterations in source-sink metabolism at both location. IITA Ibadan also does conventional cassava breeding and we work together on different projects characterizing these genotypes.
Our research group and the entire Cassava Source-Sink project hope to contribute towards food security of African smallholder farmers. If you are interested in cassava or would like to support us in any way, feel free to contact wolfgang.zierer@fau.de.
